Published in Science Advances and featured as TU Wien press release in April 2022
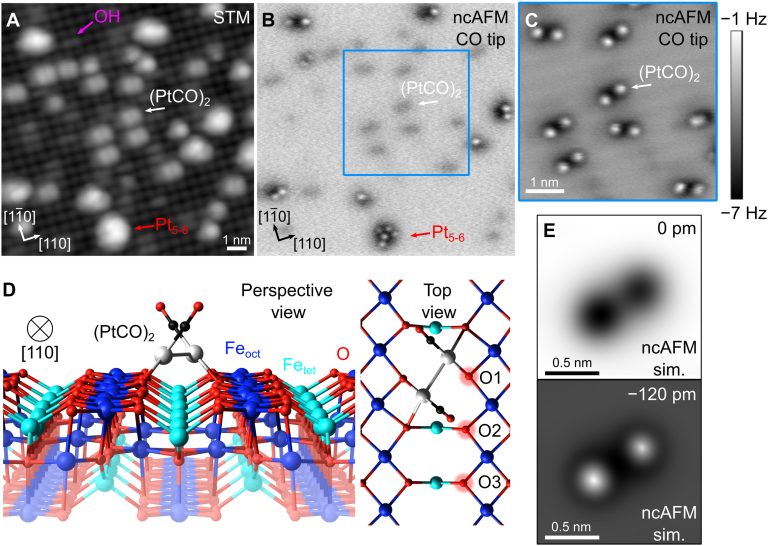
Heterogeneous catalysts based on subnanometer metal clusters often exhibit strongly size-dependent properties, and the addition or removal of a single atom can make all the difference. Identifying the most active species and deciphering the reaction mechanism is extremely difficult, however, because it is often not clear how the catalyst evolves in operando. Here, we use a combination of atomically resolved scanning probe microscopies, spectroscopic techniques, and density functional theory (DFT)–based calculations to study CO oxidation by a model Pt/Fe3O4(001) “single-atom” catalyst. We demonstrate that (PtCO)2 dimers, formed dynamically through the agglomeration of mobile Pt-carbonyl species, catalyze a reaction involving the oxide support to form CO2. Pt2 dimers produce one CO2 molecule before falling apart into two adatoms, releasing the second CO. Olattice extraction only becomes facile when both the Pt-dimer and the Fe3O4 support can access metastable configurations, suggesting that substantial, concerted rearrangements of both cluster and support must be considered for reactions occurring at elevated temperature.
Authors:
Matthias Meier, Jan Hulva, Zdenenk Jakub, Florian Kraushofer, Mislav Boobić, Roland Bliem, Martin Setvin, Michael Schmid, Ulrike Diebold, Cesare Franchinie, and Gareth S. Parkinson
Subprojects:
P02 – Surface structure and reactivity of multi-component oxides at the atomic scale
P04 – Atomic-scale studies of catalysis by spinel oxides
P07 – Polaron pattern recognition in correlated oxide surfaces