Published in Science Advances
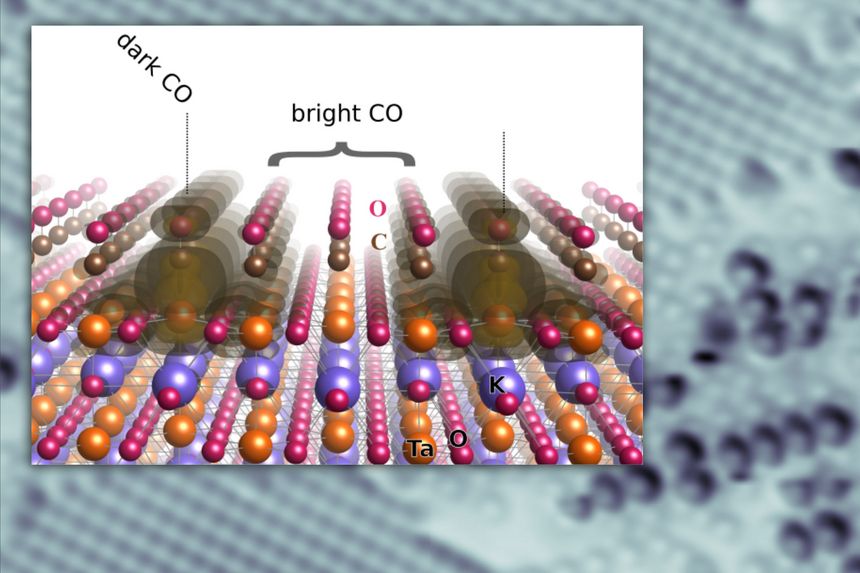
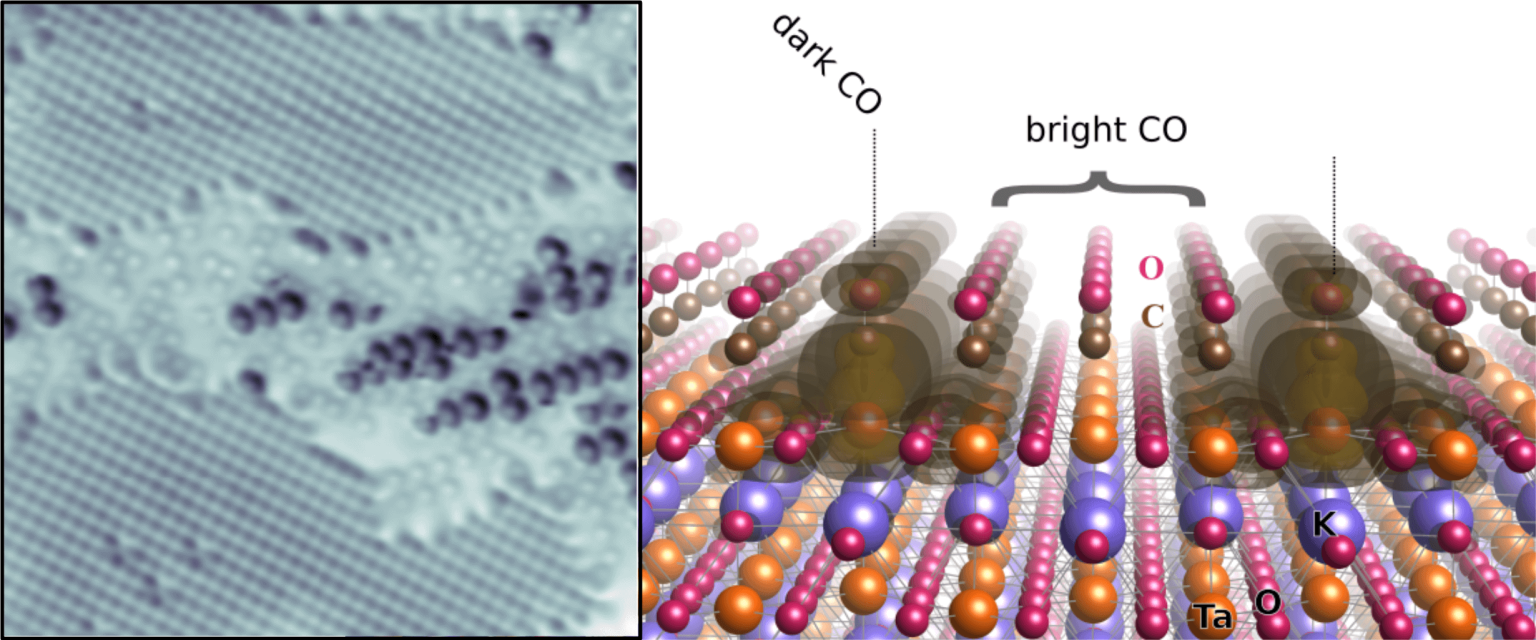
Polarizable materials attract attention in catalysis because they have a free parameter for tuning chemical reactivity. Their surfaces entangle the dielectric polarization with surface polarity, excess charge, and orbital hybridization. How this affects individual adsorbed molecules is shown for the incipient ferroelectric perovskite KTaO3. This intrinsically polar material cleaves along (001) into KO- and TaO2-terminated surface domains. At TaO2 terraces, the polarity-compensating excess electrons form a two-dimensional electron gas and can also localize by coupling to ferroelectric distortions. TaO2 terraces host two distinct types of CO molecules, adsorbed at equivalent lattice sites but charged differently as seen in atomic force microscopy/scanning tunneling microscopy. Temperature-programmed desorption shows substantially stronger binding of the charged CO; in density functional theory calculations, the excess charge favors a bipolaronic configuration coupled to the CO. These results pinpoint how adsorption states couple to ferroelectric polarization.
The full article can be found here.
Authors:
Zhichang Wang, Michele Reticcioli, Zdenek Jakub, Igor Sokolović, Matthias Meier, Lynn A. Boatner, Michael Schmid, Gareth S. Parkinso, Ulrike Diebold, Cesare Franchini, and Martin Setvin
Subprojects:
P07 – Polaron pattern recognition in correlated oxide surfaces
P02 – Surface structure and reactivity of multi-component oxides at the atomic scale
P04 – Atomic-scale studies of catalysis by spinel oxides