Surface structure and reactivity of
multi-component oxides at the atomic scale
Subproject P02
Multi-component metal oxides exhibit a plethora of stoichiometry-dependent structural phases at the surface, even if the composition of the bulk is kept the same. The long-term objective of P02 is to unravel the relationship between surface electronic and geometric structure and reactivity, to ultimately tune these materials for energy-related reactions such as the ORR. The project applies the surface science approach. We will grow well-defined, epitaxial perovskite thin films of LSFO and LSMO in a UHV-based PLD/surface science apparatus under tight control of the surface stoichiometry in the first project period. We will determine the coordinates of surface atoms quantitatively using LEED-IV in close collaboration with theoretical groups.
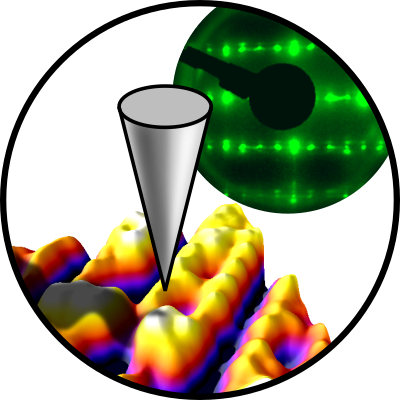
Theoretical models will also help with interpreting atomically-resolved ncAFM/STM images. These images give direct insights into the behavior of polarons in these complex materials and show how adsorbates such as O2, H2O, CO, and CO2 interact with electronic and structural defects. XPS, TPD, and FTIR of these well-defined systems will deliver desorption energies, vibrational frequencies, and spectral fingerprints. These experimental data on well-defined systems will build a bridge when tested under ‘realistic’ environments at high pressure/temperature and in aqueous solutions. They will also serve to validate ML-based theory approaches.
Expertise
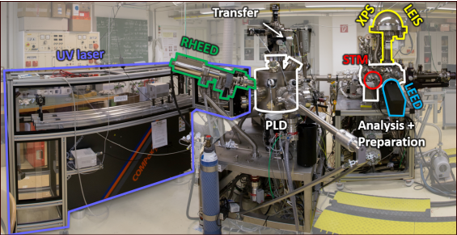
Our expertise is experimental surface science. We operate a total of seven ultrahigh-vacuum (UHV) chambers, which contain virtually all main experimental surface science techniques, as well as an (electro-)chemistry lab.
All chambers are equipped with facilities for sample preparation (sputtering/annealing/gas dosing), as well as various growth techniques (e-beam evaporators, Knudsen cells, UHV-compatible sputter deposition, pulsed laser deposition (PLD)).
Analysis techniques used in our research include:
- Scanning Tunneling Microscopy (STM) (in UHV 4K – 300 K, electrochemical STM)
- Atomic Force Microscopy (AFM): UHV-based (q+ sensor) and in the ambient (cantilever-based)
- Low-Energy Electron Diffraction (LEED)
- Reflection High Energy Diffraction (RHEED)
- X-ray Photoelectron Spectroscopy (XPS)
- Ultraviolet Photoelectron Spectroscopy (UPS)
- Auger Electron Spectroscopy (AES)
- Low-energy He+ ion scattering (LEIS)
- Thermal Programmed Desorption Spectroscopy (TPD)
Team

Former Members
Publications
Sorry, no publications matched your criteria.