Catalysis by ultrathin
LaBO3 (B=Co, Fe) perovskite films
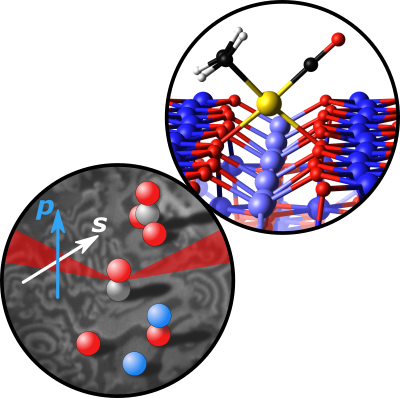
Subproject P08
Perovskites are important catalysts, but detailed knowledge of their surface structure and chemistry is often lacking. The long-term objective of P08 is to elucidate structure-function correlations and visualize molecule-perovskite interaction in reactions involving O2, H2, CO, CO2, or H2O.
In the first project period, we will develop surface science-based model systems of LaCoO3 and LaFeO3 perovskites. Both epitaxial and polycrystalline thin films will be grown in UHV, guided by characterization via LEED, SXRD, SEM/EBSD, XPS/UPS/LEIS, IRAS, and TPD. Isotopically (18O or 13C) labeled adsorbates or films will reveal how oxygen and oxygen-containing molecules are activated. We will analyze the data in close collaboration with theoretical groups who simulate structure, stability, and infrared spectra (P03 Kresse).
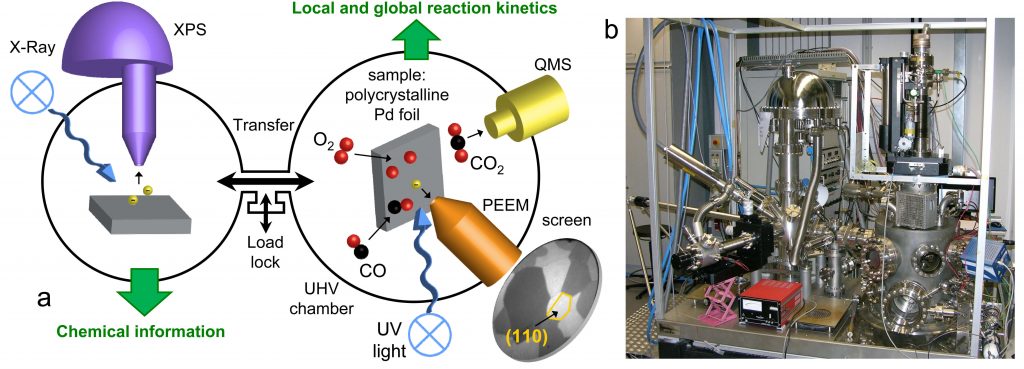
We will employ a unique combination of in situ surface spectroscopy (PM-IRAS, NAP-XPS, SXRD) and in situ surface microscopy (PEEM, SPEM), combined with MS gas phase analysis, to monitor ongoing reactions from HV to atmospheric pressure. This procedure should enable us to gain fundamental insights into the interplay of ternary oxide atomic and electronic structure, defects, composition, adsorption, as well as initiation and spatial progression of surface reactions on the mesoscale via reaction fronts (local kinetics by imaging). Project P08 will create the required bridge between single crystals (P02 Diebold, P04 Parkinson) and more application-relevant nanomaterials (P10 Föttinger).
Expertise
Our expertise is experimental surface science and its application to studies in heterogeneous catalysis. We operate a total of seven ultrahigh-vacuum (UHV) chambers, three of which are coupled to high-pressure cells. In situ and operando studies of surface reactions are carried out by area-averaging surface spectroscopy and real-time surface microscopy on the nano- and mesoscale. All chambers are equipped with facilities for sample preparation (sputtering, annealing, gas dosing), as well as various growth techniques (e-beam evaporators, Knudsen cells, sputter deposition). Analysis techniques used in our research include:
- Auger Electron Spectroscopy (AES)
- Field Emission Microscopy (FEM)
- Field Ion Microscopy (FIM)
- Gas Chromatography (GC)
- Low-Energy Electron Diffraction (LEED)
- Low-Energy Ion Scattering (LEIS)
- Mass Spectroscopy (MS)
- PhotoEmission Electron Microscopy (PEEM)
- Polarization Modulation Infrared Reflection-Absorption Spectroscopy (PM-IRAS)
- Sum Frequency Generation (SFG)
- Scanning PhotoElectron Microscopy (SPEM)
- Scanning Tunneling Microscopy (STM)
- Temperature-Programmed Desorption (TPD)
- Ultraviolet Photoelectron Spectroscopy (UPS)
- X-ray Absorption Spectroscopy (XAS)
- Surface X-Ray Diffraction (SXRD)
- X-ray Photoelectron Spectroscopy (XPS)
Collaboration Partners:
- Prof. Andreas Stierle, DESY Hamburg, Germany: SXRD
- Dr. Luca Gregoratti, ELETTRA Sincrotrone Trieste, Italy: SPEM
The combined application of photoemission electron microscopy (PEEM) and scanning photoelectron microscopy (SPEM) is particularly beneficial for TACO because these techniques allow visualizing ongoing reactions and local surface analysis on a µm-scale.
Team
Associates
Former Members
Publications
2025

Yigit, Nevzat; Föttinger, Karin; Bernardi, Johannes; Rupprechter, Günther
Journal ArticleOpen AccessIn: Journal of Catalysis, iss. 0021-9517, pp. 115973, 2025.
Abstract | Links | BibTeX | Tags: P08, P10
@article{Yigit_2024a,
title = {Preferential CO oxidation (PROX) on LaCoO_{3}–based catalysts: Effect of cobalt oxidation state on selectivity},
author = {Nevzat Yigit and Karin Föttinger and Johannes Bernardi and Günther Rupprechter},
url = {https://doi.org/10.1016/j.jcat.2025.115973},
year = {2025},
date = {2025-01-20},
urldate = {2025-01-20},
journal = {Journal of Catalysis},
issue = {0021-9517},
pages = {115973},
abstract = {The perovskite LaCoO_{3} (LCO) was used as catalyst for preferential oxidation of CO (PROX). LCO was synthesized via the modified Pechini method and characterized by X-ray diffraction (XRD), high resolution transmission electron microscopy (HRTEM), and CO_{2–} and H_{2–} temperature programmed reduction (TPR). Different reductive and oxidative pretreatments were applied to systematically vary the Co oxidation state in order to examine its effect on catalytic performance and to single out active site requirements. Upon reduction at increasing temperature, LaCoO_{3} transformed to brownmillerite-type La_{2}Co_{2}O_{5}, exsolved Co^{0} nanoparticles supported on La_{2}O_{3} and, upon reoxidation, to Co_{3}O_{4}/La_{2}O_{3}. The Co oxidation state of the various catalysts correlated with their CO_{2} selectivity: LCO containing only Co^{3+} exhibited 100 % CO_{2} selectivity in a wide temperature window, whereas La_{2}Co_{2}O_{5}, Co/La_{2}O_{3} and Co_{3}O_{4}/La_{2}O_{3} had markedly lower selectivity. It is suggested that Co^{3+} is crucial and that the strong resistivity of LaCoO_{3} towards reduction is responsible for the high and stable CO_{2} selectivity over a temperature range of 100 °C–220 °C. Higher oxygen concentration further broadens the PROX window.},
keywords = {P08, P10},
pubstate = {published},
tppubtype = {article}
}
2024
Li, Xia; Rupprechter, Günther
Journal ArticleOpen AccessIn: Surface Science Reports, vol. 79, iss. 4, pp. 100645, 2024.
Abstract | Links | BibTeX | Tags: P08
@article{Li_2024a,
title = {Sum frequency generation (SFG) spectroscopy at surfaces and interfaces: Adsorbate structure and molecular bond orientation},
author = {Xia Li and Günther Rupprechter},
url = {https://doi.org/10.1016/j.surfrep.2024.100645},
year = {2024},
date = {2024-12-05},
urldate = {2024-12-05},
journal = {Surface Science Reports},
volume = {79},
issue = {4},
pages = {100645},
abstract = {Infrared (IR)-visible (Vis) sum frequency generation (SFG) is a second-order nonlinear optical process which is forbidden in centrosymmetric bulk media or isotropic phases, but allowed at (open) surfaces or (buried) interfaces where the inversion symmetry is broken. SFG spectroscopy is thus inherently surface- or interface-specific, providing information about the structure, orientation, surface number density, chirality, and dynamics of molecules, provided the system of interest is accessible by light. This review illustrates basic SFG concepts, theory, operation modes (e.g., frequency-domain, broadband, homodyne/heterodyne, time-resolved), and recent extensions and developments of SFG (e.g., doubly resonant, plasmon-enhanced, chiral, microscopy). To illustrate the wide range of SFG applications, selected case studies discuss the characterization of molecular structure and bond orientation at solid-gas, solid-liquid, liquid-air, liquid-liquid, and solid-solid interfaces.},
keywords = {P08},
pubstate = {published},
tppubtype = {article}
}
Tangpakonsab, Parinya; Genest, Alexander; Parkinson, Gareth S.; Rupprechter, Günther
Journal ArticleOpen AccessSubmittedIn: ChemRxiv, 2024.
Abstract | Links | BibTeX | Tags: P04, P08
@article{Tangpakonsab_2024a,
title = {Mechanistic Insights into CO and H_{2} Oxidation on Cu/CeO_{2} Single Atom Catalysts: A Computational Investigation},
author = {Parinya Tangpakonsab and Alexander Genest and Gareth S. Parkinson and Günther Rupprechter},
url = {https://chemrxiv.org/engage/chemrxiv/article-details/673325d25a82cea2fadefd76},
year = {2024},
date = {2024-11-14},
urldate = {2024-11-14},
journal = {ChemRxiv},
abstract = {Single atom catalysts (SACs) have attracted significant interest due to their unique properties and potential for enhancing catalytic performance in various chemical reactions. In this study, we atomistically explore adsorption properties and catalytic performance of single Cu atoms anchored at low-index CeO_{2} surfaces, focusing on the oxidation of CO and H_{2}. Utilizing density functional theory (DFT) calculations, we report that Cu adatoms bind favorably on different CeO_{2} surfaces, following a stability order of (100)>(110)>(111). The charge transfer from a single adsorbed Cu atom to Ce leads to the reduction of Ce^{4+} to Ce^{3+} and the oxidation of Cu^{0} to Cu^{+}. This strengthens molecular bonds at Cu sites, particularly for CO due to the less populated d-band, while H_{2} shows a by ~1 eV weaker adsorption. CO oxidation is energetically more favorable than H_{2} oxidation on the Cu/CeO_{2}(111) surface. The rate-controlling steps for the Mars-van Krevelen oxidation involve the formation of a bent CO_{2}^{-} intermediate for CO and H_{2}O for H_{2}. The lattice oxygen atom at the interface plays a key role for both oxidation processes. Our findings highlight the potential of single atom catalyst, Cu/CeO_{2}, for CO adsorption and oxidation in heterogeneous catalysis.},
keywords = {P04, P08},
pubstate = {published},
tppubtype = {article}
}

Haunold, Thomas; Anić, Krešimir; Genest, Alexander; Rameshan, Christoph; Roiaz, Matteo; Li, Hao; Wicht, Thomas; Knudsen, Jan; Rupprechter, Günther
Journal ArticleOpen AccessIn: Surface Science, 2024.
Abstract | Links | BibTeX | Tags: P08, P10
@article{Haunold_2024a,
title = {Hydroxylation of an ultrathin Co_{3}O_{4}(111) film on Ir(100) studied by in situ ambient pressure XPS and DFT},
author = {Thomas Haunold and Krešimir Anić and Alexander Genest and Christoph Rameshan and Matteo Roiaz and Hao Li and Thomas Wicht and Jan Knudsen and Günther Rupprechter},
url = {https://doi.org/10.1016/j.susc.2024.122618},
year = {2024},
date = {2024-09-26},
urldate = {2024-09-26},
journal = {Surface Science},
abstract = {In the present work, we have studied the interaction of water with spinel cobalt oxide (Co_{3}O_{4}), an effect which has been considered a major cause of its catalytic deactivation. Employing a Co_{3}O_{4}(111) model thin film grown on Ir(100) in (ultra)high vacuum, and ambient pressure X-ray photoelectron spectroscopy (APXPS), hydroxylation in 0.5 mbar H_{2}O vapor at room temperature was monitored in real time. The surface hydroxyl (OH) coverage was determined via two different models based (i) on the termination of a pristine and OH-covered Co_{3}O_{4}(111) surface as derived from density functional theory (DFT) calculations, and (ii) on a homogeneous cobalt oxyhydroxide (CoO(OH)) overlayer. Langmuir pseudo-second-order kinetics were applied to characterize the OH evolution with time, suggesting two regimes of chemisorption at the mosaic-like Co_{3}O_{4}(111) film: (i) plateaus, which were quickly saturated by OH, followed by (ii) slow hydroxylation in the “cracks” of the thin film. H_{2}O dissociation and OH formation, blocking exposed Co^{2+} ions and additionally consuming surface lattice oxygen, respectively, may thus account for catalyst deactivation by H_{2}O traces in reactive feeds.},
keywords = {P08, P10},
pubstate = {published},
tppubtype = {article}
}

Maqbool, Qaisar; Dobrezberger, Klaus; Stropp, Julian; Huber, Martin; Kontrus, Karl-Leopold; Aspalter, Anna; Neuhauser, Julie; Schachinger, Thomas; Löffler, Stefan; Rupprechter, Günther
Journal ArticleOpen AccessIn: RSC Sustainability, vol. 2, iss. 11, pp. 3276–3288, 2024.
Abstract | Links | BibTeX | Tags: P08
@article{Maqbool_2024a,
title = {Bimetallic CuPd nanoparticles supported on ZnO or graphene for CO_{2} and CO conversion to methane and methanol},
author = {Qaisar Maqbool and Klaus Dobrezberger and Julian Stropp and Martin Huber and Karl-Leopold Kontrus and Anna Aspalter and Julie Neuhauser and Thomas Schachinger and Stefan Löffler and Günther Rupprechter},
url = {https://doi.org/10.1039/D4SU00339J },
doi = {10.1039/D4SU00339J},
year = {2024},
date = {2024-09-04},
urldate = {2024-09-04},
journal = {RSC Sustainability},
volume = {2},
issue = {11},
pages = {3276--3288},
abstract = {Carbon dioxide (CO_{2}) and carbon monoxide (CO) hydrogenation to methane (CH_{4}) or methanol (MeOH) is a promising pathway to reduce CO_{2} emissions and to mitigate dependence on rapidly depleting fossil fuels. Along these lines, a series of catalysts comprising copper (Cu) or palladium (Pd) nanoparticles (NPs) supported on zinc oxide (ZnO) as well as bimetallic CuPd NPs supported on ZnO or graphene were synthesized via various methodologies. The prepared catalysts underwent comprehensive characterization via high-resolution transmission electron microscopy (HRTEM), energy-dispersive X-ray spectroscopy (EDX) mapping, electron energy loss spectroscopy (EELS), X-ray diffraction (XRD), hydrogen temperature-programmed reduction and desorption (H_{2}-TPR and H_{2}-TPD), and deuterium temperature-programmed desorption (D_{2}O-TPD). In the CO2 hydrogenation process carried out at 20 bar and elevated temperatures (300 to 500 °C), Cu, Pd, and CuPd NPs (<5 wt% loading) supported on ZnO or graphene predominantly yielded CH_{4} as the primary product, with CO generated as a byproduct via the reverse water gas shift (RWGS) reaction. For CO hydrogenation between 400 and 500 °C, the CO conversion was at least 40% higher than the CO_{2} conversion, with CH_{4} and CO_{2} identified as the main products, the latter from water gas shift. Employing 90 wt% Cu on ZnO led to an enhanced CO conversion of 14%, with the MeOH yield reaching 10% and the CO_{2} yield reaching 4.3% at 230 °C. Overall, the results demonstrate that lower Cu/Pd loading (<5 wt%) supported on ZnO/graphene favored CH_{4} production, while higher Cu content (90 wt%) promoted MeOH production, for both CO_{2} and CO hydrogenation at high pressure.},
keywords = {P08},
pubstate = {published},
tppubtype = {article}
}

Amati, Matteo; Yashina, Lada V.; Winkler, Philipp; Sparwasser, Kevin; Milosz, Zygmunt; Rupprechter, Günther; Gregoratti, Luca
Catalytically Active Materials Visualized by Scanning Photoelectron Spectro-Microscopy
Journal ArticleOpen AccessIn: Surfaces, vol. 7, pp. 442–459, 2024.
Abstract | Links | BibTeX | Tags: P08
@article{nokey,
title = {Catalytically Active Materials Visualized by Scanning Photoelectron Spectro-Microscopy},
author = {Matteo Amati and Lada V. Yashina and Philipp Winkler and Kevin Sparwasser and Zygmunt Milosz and Günther Rupprechter and Luca Gregoratti},
doi = {10.3390/surfaces7030028},
year = {2024},
date = {2024-06-26},
journal = {Surfaces},
volume = {7},
pages = {442--459},
abstract = {Modern catalysts are complex systems whose performance depends both on space and time domains and, most importantly, on the operational environment. As a direct consequence, understanding their functionalities requires sophisticated techniques and tools for measurement and simulation, addressing the proper spatial and temporal scale and being capable of mimicking the working conditions of every single component, such as catalyst supports, electrodes, electrolytes, as well as of the entire assembly, e.g., in the case of fuel cells or batteries. Scanning photoelectron spectro-microscopy (SPEM) is one of the approaches that allow combining X-ray photoelectron spectroscopy with sub-micron spatial resolution; in particular, the SPEM hosted at the ESCA Microscopy beamline at Elettra has been upgraded to conduct in situ and operando experiments. Three different case studies are presented to illustrate the capabilities of the SPEM in the investigation of catalytic materials in different conditions and processes.},
keywords = {P08},
pubstate = {published},
tppubtype = {article}
}

Sringam, Sarannuch; Witoon, Thongthai; Wattanakit, Chularat; Donphai, Waleeporn; Chareonpanich, Metta; Rupprechter, Günther; Seubsai, Anusorn
Journal ArticleOpen AccessIn: Carbon Resources Conversion, pp. 100261, 2024, ISSN: 2588-9133.
Abstract | Links | BibTeX | Tags: P08
@article{Sringam_2024a,
title = {Effect of calcination temperature on the performance of K-Co/Al_{2}O_{3} catalyst for oxidative coupling of methane},
author = {Sarannuch Sringam and Thongthai Witoon and Chularat Wattanakit and Waleeporn Donphai and Metta Chareonpanich and Günther Rupprechter and Anusorn Seubsai},
url = {https://doi.org/10.1016/j.crcon.2024.100261},
doi = {10.1016/j.crcon.2024.100261},
issn = {2588-9133},
year = {2024},
date = {2024-05-28},
journal = {Carbon Resources Conversion},
pages = {100261},
abstract = {The oxidative coupling of methane (OCM) involves directly converting methane to C_{2+} hydrocarbons (such as ethylene and ethane) via a reaction with oxygen. This study elucidated the effect of the calcination temperature on the structure and catalytic performance of potassium-doped-cobalt oxide supported on an alumina (K-Co/Al_{2}O_{3}) catalyst for the OCM reaction. The catalyst was highly active at relatively low reactor temperatures (500–640 °C). Four calcination temperatures (400, 500, 600, and 700 °C) were investigated, with the results showing that the calcination temperature strongly affected catalytic properties, such as the crystalline phases, elemental distribution, physical properties, and catalytic basicity, leading to a wide range in catalytic performances. The catalyst calcined at 400 °C was superior among the catalysts, with 8.3 % C_{2+} yield, 24.8 % C_{2+} selectivity, and 33.6 % CH_{4} conversion at 640 °C. Furthermore, the catalyst was robust over 24 h of testing.},
keywords = {P08},
pubstate = {published},
tppubtype = {article}
}

Maqbool, Qaisar; Favoni, Orlando; Wicht, Thomas; Lasemi, Niusha; Sabbatini, Simona; Stöger-Pollach, Michael; Ruello, Maria Letizia; Tittarelli, Francesca; Rupprechter, Günther
Highly Stable Self-Cleaning Paints Based on Waste-Valorized PNC-Doped TiO2 Nanoparticles
Journal ArticleOpen AccessIn: ACS Catalysis, vol. 14, iss. 7, pp. 4820–4834, 2024.
Abstract | Links | BibTeX | Tags: P08
@article{Maqbool_2024b,
title = {Highly Stable Self-Cleaning Paints Based on Waste-Valorized PNC-Doped TiO_{2} Nanoparticles},
author = {Qaisar Maqbool and Orlando Favoni and Thomas Wicht and Niusha Lasemi and Simona Sabbatini and Michael Stöger-Pollach and Maria Letizia Ruello and Francesca Tittarelli and Günther Rupprechter},
url = {https://doi.org/10.1021/acscatal.3c06203},
year = {2024},
date = {2024-03-15},
journal = {ACS Catalysis},
volume = {14},
issue = {7},
pages = {4820–4834},
abstract = {Adding photocatalytically active TiO_{2} nanoparticles (NPs) to polymeric paints is a feasible route toward self-cleaning coatings. While paint modification by TiO_{2}-NPs may improve photoactivity, it may also cause polymer degradation and release of toxic volatile organic compounds. To counterbalance adverse effects, a synthesis method for nonmetal (P, N, and C)-doped TiO_{2}-NPs is introduced, based purely on waste valorization. PNC-doped TiO_{2}-NP characterization by vibrational and photoelectron spectroscopy, electron microscopy, diffraction, and thermal analysis suggests that TiO_{2}-NPs were modified with phosphate (P═O), imine species (R═N-R), and carbon, which also hindered the anatase/rutile phase transformation, even upon 700 °C calcination. When added to water-based paints, PNC-doped TiO_{2}-NPs achieved 96% removal of surface-adsorbed pollutants under natural sunlight or UV, paralleled by stability of the paint formulation, as confirmed by micro-Fourier transform infrared (FTIR) surface analysis. The origin of the photoinduced self-cleaning properties was rationalized by three-dimensional (3D) and synchronous photoluminescence spectroscopy, indicating that the dopants led to 7.3 times stronger inhibition of photoinduced e^{–}/h^{+} recombination when compared to a benchmark P25 photocatalyst.},
keywords = {P08},
pubstate = {published},
tppubtype = {article}
}

Sidorowicz, Agnieszka; Yigit, Nevzat; Wicht, Thomas; Stöger-Pollach, Michael; Concas, Alessandro; Orrù, Roberto; Cao, Giacomo; Rupprechter, Günther
Microalgae-derived Co3O4 nanomaterials for catalytic CO oxidation
Journal ArticleOpen AccessIn: RSC Advances, vol. 14, pp. 4575-4586, 2024.
Abstract | Links | BibTeX | Tags: P08
@article{Sidorowicz_2024a,
title = {Microalgae-derived Co_{3}O_{4} nanomaterials for catalytic CO oxidation},
author = {Agnieszka Sidorowicz and Nevzat Yigit and Thomas Wicht and Michael Stöger-Pollach and Alessandro Concas and Roberto Orrù and Giacomo Cao and Günther Rupprechter},
url = {https://doi.org/10.1039/D4RA00343H},
year = {2024},
date = {2024-02-05},
urldate = {2024-02-05},
journal = {RSC Advances},
volume = {14},
pages = {4575-4586},
abstract = {Efficient carbon monoxide oxidation is important to reduce its impacts on both human health and the environment. Following a sustainable synthesis route toward new catalysts, nanosized Co_{3}O_{4} was synthesized based on extracts of microalgae: Spirulina platensis, Chlorella vulgaris, and Haematococcus pluvialis. Using the metabolites in the extract and applying different calcination temperatures (450, 650, 800 °C) led to Co_{3}O_{4} catalysts with distinctly different properties. The obtained Co_{3}O_{4} nanomaterials exhibited octahedral, nanosheet, and spherical morphologies with structural defects and surface segregation of phosphorous and potassium, originating from the extracts. The presence of P and K in the oxide nanostructures significantly improved their catalytic CO oxidation activity. When normalized by the specific surface area, the microalgae-derived catalysts exceeded a commercial benchmark catalyst. In situ studies revealed differences in oxygen mobility and carbonate formation during the reaction. The obtained insights may facilitate the development of new synthesis strategies for manufacturing highly active Co_{3}O_{4} nanocatalysts.},
keywords = {P08},
pubstate = {published},
tppubtype = {article}
}

Chanka, Napassorn; Donphai, Waleeporn; Chareonpanich, Metta; Faungnawakij, Kajornsak; Rupprechter, Günther; Seubsai, Anusorn
Journal ArticleOpen AccessIn: ACS Omega, vol. 9, iss. 6, pp. 6749–6760, 2024.
Abstract | Links | BibTeX | Tags: P08
@article{Chanka_2024a,
title = {Potassium Permanganate-Impregnated Amorphous Silica–Alumina Derived from Sugar Cane Bagasse Ash as an Ethylene Scavenger for Extending Shelf Life of Mango Fruits},
author = {Napassorn Chanka and Waleeporn Donphai and Metta Chareonpanich and Kajornsak Faungnawakij and Günther Rupprechter and Anusorn Seubsai},
url = {https://doi.org/10.1021/acsomega.3c08119},
year = {2024},
date = {2024-02-02},
journal = {ACS Omega},
volume = {9},
issue = {6},
pages = {6749–6760},
abstract = {Ethylene, a plant hormone, is a gas that plays a crucial role in fruit ripening and senescence. In this work, a novel ethylene scavenger was prepared from amorphous silica–alumina derived from sugar cane bagasse ash (SC-ASA) and used to prolong the shelf life of mango fruits during storage. KMnO_{4} at 2, 4, or 6 wt %/w was loaded on SC-ASA using an impregnation method. The results showed that 4% w/w KMnO_{4} loaded on SC-ASA (4KM/SC-ASA) was superior for ethylene removal at an initial ethylene concentration of 400 μL L^{–1} for 120 min under ambient conditions (25–27 °C and 70–75% relative humidity), resulting in 100% ethylene removal. The kinetic study of ethylene removal showed that the adsorption data were best fitted with a pseudo-first-order kinetic model. The effects of 4KM/SC-ASA as sachets on the quality changes of the mango fruits were investigated, with the results showing that mango fruits packed in cardboard boxes with 4KM/SC-ASA had significantly delayed ripening, low levels of ethylene production, respiration, and weight loss, high fruit firmness, low total soluble solids, and high acidity compared to those of the control treatment. These findings should contribute to developing an ethylene scavenger to extend the shelf life of fruits, reduce the waste of the sugar and ethanol industries, and make it a valuable material.},
keywords = {P08},
pubstate = {published},
tppubtype = {article}
}