Fundamentals of the structure of
liquid/oxide interfaces
Subproject P11
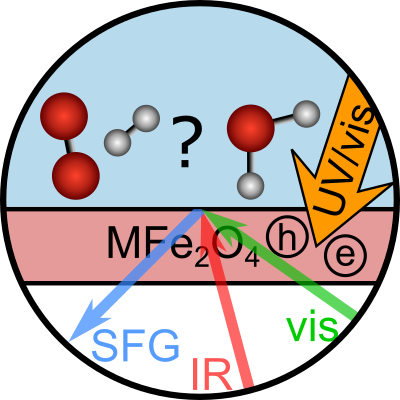
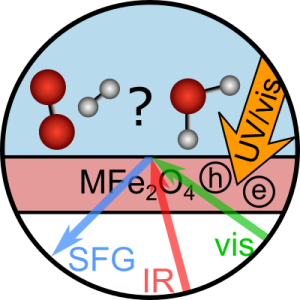
Obtaining mechanistic insights into photocatalytic processes in their natural aqueous environment is challenging since it is difficult to probe just the interfacial molecules in the presence of the bulk. Moreover, typical reaction timescales are very short, making high time resolution necessary.
The long-term objective of P11 is to unravel the reaction mechanism of photocatalytic processes in real-time directly at the water-catalyst interface, where the reaction is taking place. In the first project period, we focus on photocatalytic water splitting at iron spinel oxide interfaces. The project will benefit from high-quality and well-characterized spinel samples prepared by P04 Parkinson. We will use vibrational spectroscopy to probe only the interfacial molecules. In this way, we study the strength of hydrogen bonds and the orientation of the water molecules, i.e., how water binds to various spinel oxides. Subsequently, photodissociation will be initiated by a laser pulse mimicking sunlight. We will follow the reaction on sub-picosecond timescales by probing the water molecules and potential reaction intermediates and products. The collaboration with theoretical projects helps with data interpretation and fundamental insights into the reaction (P12 Dellago). The water structure and reaction dynamics will be linked to reported efficiencies of the photocatalysts to connect molecular-level details to high catalytic efficiency (P10 Föttinger).
Expertise
Our expertise is ultrafast and nonlinear optical spectroscopy, predominantly in the infrared spectral region. We have two femtosecond amplifiers and several optical parametric amplifiers to convert the amplifier’s 800 nm output into other frequencies.
To study the solid-liquid interface, our focus in the TACO project, we will have two time-resolved sum-frequency generation setups, of which one is phase-resolved as well.
Team

Associates
Former Members
Publications
2024

Romano, Salvatore; Kaur, Harsharan; Zelenka, Moritz; Hijes, Pablo Montero De; Eder, Moritz; Parkinson, Gareth S.; Backus, Ellen H. G.; Dellago, Christoph
Journal ArticleOpen AccessSubmittedarXivIn: arXiv, 2024.
Abstract | Links | BibTeX | Tags: P04, P11, P12
@article{Romano_2024b,
title = {Structure of the water/magnetite interface from sum frequency generation experiments and neural network based molecular dynamics simulations},
author = {Salvatore Romano and Harsharan Kaur and Moritz Zelenka and Pablo Montero De Hijes and Moritz Eder and Gareth S. Parkinson and Ellen H. G. Backus and Christoph Dellago},
url = {https://arxiv.org/abs/2410.12717},
year = {2024},
date = {2024-10-16},
urldate = {2024-10-16},
journal = {arXiv},
abstract = {Magnetite, a naturally abundant mineral, frequently interacts with water in both natural settings and various technical applications, making the study of its surface chemistry highly relevant. In this work, we investigate the hydrogen bonding dynamics and the presence of hydroxyl species at the magnetite-water interface using a combination of neural network potential-based molecular dynamics simulations and sum frequency generation vibrational spectroscopy. Our simulations, which involved large water systems, allowed us to identify distinct interfacial species, such as dissociated hydrogen and hydroxide ions formed by water dissociation. Notably, water molecules near the interface exhibited a preference for dipole orientation towards the surface, with bulk-like water behavior only re-emerging beyond 60 Å from the surface. The vibrational spectroscopy results aligned well with the simulations, confirming the presence of a hydrogen bond network in the surface ad-layers. The analysis revealed that surface-adsorbed hydroxyl groups orient their hydrogen atoms towards the water bulk. In contrast, hydrogen-bonded water molecules align with their hydrogen atoms pointing towards the magnetite surface.},
keywords = {P04, P11, P12},
pubstate = {published},
tppubtype = {article}
}
2023
Backus, Ellen H. G.; Hosseinpour, Saman; Ramanan, Charusheela; Sun, Shumei; Schlegel, Simon J.; Zelenka, Moritz; Jia, Xiaoyu; Gebhard, Maximilian; Devi, Anjana; Wang, Hai I.; Bonn, Mischa
Journal ArticleOpen AccessIn: Angewandte Chemie - International Edition, no. e202312123, 2023, ISSN: 1521-3773.
Abstract | Links | BibTeX | Tags: P11
@article{Backus2024,
title = {Ultrafast Surface‐Specific Spectroscopy of Water at a Photoexcited TiO2 Model Water‐Splitting Photocatalyst},
author = {Ellen H. G. Backus and Saman Hosseinpour and Charusheela Ramanan and Shumei Sun and Simon J. Schlegel and Moritz Zelenka and Xiaoyu Jia and Maximilian Gebhard and Anjana Devi and Hai I. Wang and Mischa Bonn},
doi = {10.1002/anie.202312123},
issn = {1521-3773},
year = {2023},
date = {2023-11-27},
urldate = {2023-11-27},
journal = {Angewandte Chemie - International Edition},
number = {e202312123},
publisher = {Wiley},
abstract = {A critical step in photocatalytic water dissociation is the hole-mediated oxidation reaction. Molecular-level insights into the mechanism of this complex reaction under realistic conditions with high temporal resolution are highly desirable. Here, we use femtosecond time-resolved, surface-specific vibrational sum frequency generation spectroscopy to study the photo-induced reaction directly at the interface of the photocatalyst TiO_{2} in contact with liquid water at room temperature. Thanks to the inherent surface specificity of the spectroscopic method, we can follow the reaction of solely the interfacial water molecules directly at the interface at timescales on which the reaction takes place. Following the generation of holes at the surface immediately after photoexcitation of the catalyst with UV light, water dissociation occurs on a sub-20 ps timescale. The reaction mechanism is similar at pH 3 and 11. In both cases, we observe the conversion of H_{2}O into Ti−OH groups and the deprotonation of pre-existing Ti−OH groups. This study provides unique experimental insights into the early steps of the photo-induced dissociation processes at the photocatalyst-water interface, relevant to the design of improved photocatalysts.},
keywords = {P11},
pubstate = {published},
tppubtype = {article}
}

Buessler, Martin; Maruyama, Shingo; Zelenka, Moritz; Onishi, Hiroshi; Backus, Ellen H. G.
Journal ArticleOpen AccessIn: Physical Chemistry Chemical Physics, vol. 25, pp. 31471–31480, 2023.
Abstract | Links | BibTeX | Tags: P11
@article{Buessler2023,
title = {Unravelling the interfacial water structure at the photocatalyst strontium titanate by sum frequency generation spectroscopy},
author = {Martin Buessler and Shingo Maruyama and Moritz Zelenka and Hiroshi Onishi and Ellen H. G. Backus},
doi = {https://doi.org/10.1039/D3CP03829G},
year = {2023},
date = {2023-10-31},
urldate = {2023-10-31},
journal = {Physical Chemistry Chemical Physics},
volume = {25},
pages = {31471--31480},
abstract = {The direct conversion of solar energy to hydrogen is considered as a possible method to produce carbon neutral hydrogen fuel. The mechanism of photocatalytic water splitting involves the chemical breakdown of water and re-assembly into hydrogen and oxygen at the interface of a photocatalyst. The selection rules of a suitable material are well established, but the fundamental understanding of the mechanisms, occurring at the interface between the catalyst and the water, remains missing. Using surface specific sum frequency generation spectroscopy, we present here characterisation of the interface between water and the photocatalyst strontium titanate (SrTiO_{3}). We monitor the OH-stretching vibrations present at the interface. Their variations of intensities and frequencies as functions of isotopic dilution, pH and salt concentration provide information about the nature of the hydrogen bonding environment. We observe the presence of water molecules that flip their orientation at pH 5 indicating the point of zero charge of the SrTiO_{3} layer. These water molecules are oriented with their hydrogen away from the surface when the pH of the solutions is below 5 and pointing towards the surface when the pH is higher than 5. Besides, water molecules donating a H-bond to probably surface TiOH groups are observed at all pH.},
keywords = {P11},
pubstate = {published},
tppubtype = {article}
}
Zelenka, Moritz; Backus, Ellen H. G.
Investigating aqueous mineral interfaces using sum frequency generation spectroscopy
Book ChapterIn: Wandelt, Klaus; Bussetti, Gianlorenzo (Ed.): pp. 148-157, Elsevier, Oxford, Encyclopedia of Solid-Liquid Interfaces, 2023.
Abstract | Links | BibTeX | Tags: P11
@inbook{Zelenka_2024,
title = {Investigating aqueous mineral interfaces using sum frequency generation spectroscopy},
author = {Moritz Zelenka and Ellen H. G. Backus},
editor = {Klaus Wandelt and Gianlorenzo Bussetti},
url = {https://doi.org/10.1016/B978-0-323-85669-0.00016-7
https://www.sciencedirect.com/science/article/pii/B9780323856690000167},
doi = {10.1016/B978-0-323-85669-0.00016-7},
year = {2023},
date = {2023-08-30},
urldate = {2023-08-30},
pages = {148-157},
publisher = {Elsevier},
address = {Oxford},
edition = {Encyclopedia of Solid-Liquid Interfaces},
abstract = {Sum frequency generation spectroscopy can be utilized to address the challenge of studying solid-liquid interfaces under ambient pressure and with macroscopic amounts of liquid, while still retrieving molecular level information. By utilizing a non-linear optical process, this method allows to selectively measure vibrations of interfacial species. Within this article sum frequency generation spectroscopy is first briefly introduced and then comprehensively evaluated as an investigative tool for solid-liquid interfaces, with mineral-water interfaces as model system. Additionally, it is explained how the actual probing depth of this method depends on both the experimental setup and the properties of the investigated system, such as surface charges.},
keywords = {P11},
pubstate = {published},
tppubtype = {inbook}
}