Catalysis by ultrathin
LaBO3 (B=Co, Fe) perovskite films
Subproject P08
Perovskites are important catalysts, but detailed knowledge of their surface structure and chemistry is often lacking. The long-term objective of P08 is to elucidate structure-function correlations and visualize molecule-perovskite interaction in reactions involving O2, H2, CO, CO2, or H2O.
In the first project period, we will develop surface science-based model systems of LaCoO3 and LaFeO3 perovskites. Both epitaxial and polycrystalline thin films will be grown in UHV, guided by characterization via LEED, SXRD, SEM/EBSD, XPS/UPS/LEIS, IRAS, and TPD. Isotopically (18O or 13C) labeled adsorbates or films will reveal how oxygen and oxygen-containing molecules are activated. We will analyze the data in close collaboration with theoretical groups who simulate structure, stability, and infrared spectra (P03 Kresse).
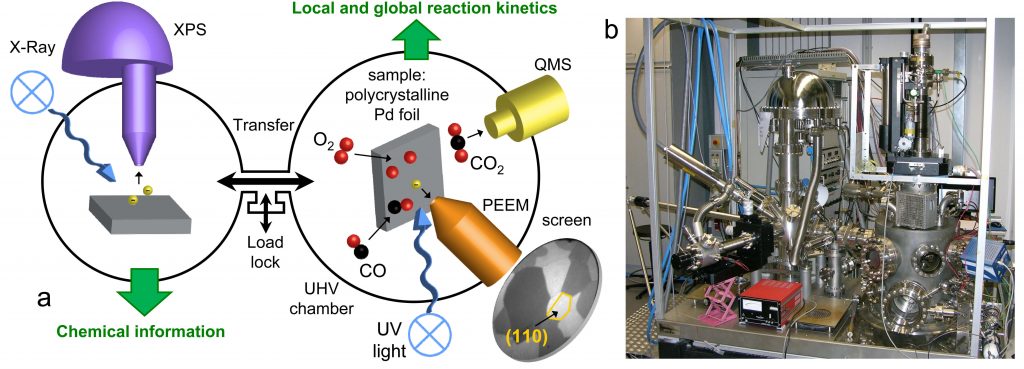
We will employ a unique combination of in situ surface spectroscopy (PM-IRAS, NAP-XPS, SXRD) and in situ surface microscopy (PEEM, SPEM), combined with MS gas phase analysis, to monitor ongoing reactions from HV to atmospheric pressure. This procedure should enable us to gain fundamental insights into the interplay of ternary oxide atomic and electronic structure, defects, composition, adsorption, as well as initiation and spatial progression of surface reactions on the mesoscale via reaction fronts (local kinetics by imaging). Project P08 will create the required bridge between single crystals (P02 Diebold, P04 Parkinson) and more application-relevant nanomaterials (P10 Föttinger).
Expertise
Our expertise is experimental surface science and its application to studies in heterogeneous catalysis. We operate a total of seven ultrahigh-vacuum (UHV) chambers, three of which are coupled to high-pressure cells. In situ and operando studies of surface reactions are carried out by area-averaging surface spectroscopy and real-time surface microscopy on the nano- and mesoscale. All chambers are equipped with facilities for sample preparation (sputtering, annealing, gas dosing), as well as various growth techniques (e-beam evaporators, Knudsen cells, sputter deposition). Analysis techniques used in our research include:
- Auger Electron Spectroscopy (AES)
- Field Emission Microscopy (FEM)
- Field Ion Microscopy (FIM)
- Gas Chromatography (GC)
- Low-Energy Electron Diffraction (LEED)
- Low-Energy Ion Scattering (LEIS)
- Mass Spectroscopy (MS)
- PhotoEmission Electron Microscopy (PEEM)
- Polarization Modulation Infrared Reflection-Absorption Spectroscopy (PM-IRAS)
- Sum Frequency Generation (SFG)
- Scanning PhotoElectron Microscopy (SPEM)
- Scanning Tunneling Microscopy (STM)
- Temperature-Programmed Desorption (TPD)
- Ultraviolet Photoelectron Spectroscopy (UPS)
- X-ray Absorption Spectroscopy (XAS)
- Surface X-Ray Diffraction (SXRD)
- X-ray Photoelectron Spectroscopy (XPS)
Collaboration Partners:
- Prof. Andreas Stierle, DESY Hamburg, Germany: SXRD
- Dr. Luca Gregoratti, ELETTRA Sincrotrone Trieste, Italy: SPEM
The combined application of photoemission electron microscopy (PEEM) and scanning photoelectron microscopy (SPEM) is particularly beneficial for TACO because these techniques allow visualizing ongoing reactions and local surface analysis on a µm-scale.
Team
Associates
Former Members
Publications
2022
Yigit, Nevzat; Genest, Alexander; Terloev, Schamil; Möller, Jury; Rupprechter, Günther
Journal ArticleOpen AccessIn: Journal of Physics: Condensed Matter, vol. 34, no. 35, pp. 354001, 2022.
Abstract | Links | BibTeX | Tags: P08
@article{Yigit2022,
title = {Active sites and deactivation of room temperature CO oxidation on Co_{3}O_{4} catalysts: combined experimental and computational investigations},
author = {Nevzat Yigit and Alexander Genest and Schamil Terloev and Jury Möller and Günther Rupprechter},
doi = {10.1088/1361-648x/ac718b},
year = {2022},
date = {2022-06-29},
urldate = {2022-06-29},
journal = {Journal of Physics: Condensed Matter},
volume = {34},
number = {35},
pages = {354001},
publisher = {IOP Publishing},
abstract = {Co_{3}O_{4} is a well-known low temperature CO oxidation catalyst, but it often suffers from deactivation. We have thus examined room temperature (RT) CO oxidation on Co_{3}O_{4} catalysts by operando DSC, TGA and MS measurements, as well as by pulsed chemisorption to differentiate the contributions of CO adsorption and reaction to CO_{2}. Catalysts pretreated in oxygen at 400 °C are most active, with the initial interaction of CO and Co_{3}O_{4} being strongly exothermic and with maximum amounts of CO adsorption and reaction. The initially high RT activity then levels-off, suggesting that the oxidative pretreatment creates an oxygen-rich reactive Co_{3}O_{4} surface that upon reaction onset loses its most active oxygen. This specific active oxygen is not reestablished by gas phase O_{2} during the RT reaction. When the reaction temperature is increased to 150 °C, full conversion can be maintained for 100 h, and even after cooling back to RT. Apparently, deactivating species are avoided this way, whereas exposing the active surface even briefly to pure CO leads to immediate deactivation. Computational modeling using DFT helped to identify the CO adsorption sites, determine oxygen vacancy formation energies and the origin of deactivation. A new species of CO bonded to oxygen vacancies at RT was identified, which may block a vacancy site from further reaction unless CO is removed at higher temperature. The interaction between oxygen vacancies was found to be small, so that in the active state several lattice oxygen species are available for reaction in parallel.},
keywords = {P08},
pubstate = {published},
tppubtype = {article}
}

Tiyatha, Worapinit; Chukeaw, Thanaphat; Sringam, Sarannuch; Witoon, Thongthai; Chareonpanich, Metta; Rupprechter, Günther; Seubsai, Anusorn
Journal ArticleOpen AccessIn: Scientific Reports, vol. 12, pp. 2595, 2022.
Abstract | Links | BibTeX | Tags: P08, TACO-associated
@article{Tiyatha2022,
title = {Oxidative coupling of methane—comparisons of MnTiO_{3}–Na_{2}WO_{4} and MnO_{x}–TiO_{2}–Na_{2}WO_{4} catalysts on different silica supports},
author = {Worapinit Tiyatha and Thanaphat Chukeaw and Sarannuch Sringam and Thongthai Witoon and Metta Chareonpanich and Günther Rupprechter and Anusorn Seubsai},
url = {https://www.nature.com/articles/s41598-022-06598-6#citeas},
doi = {10.1038/s41598-022-06598-6},
year = {2022},
date = {2022-02-16},
urldate = {2022-02-16},
journal = {Scientific Reports},
volume = {12},
pages = {2595},
publisher = {Springer Science and Business Media LLC},
abstract = {The oxidative coupling of methane (OCM) converts CH_{4} to value-added chemicals (C_{2+}), such as olefins and paraffin. For a series of MnTiO_{3}-Na_{2}WO_{4} (MnTiO_{3}-NW) and MnO_{x}-TiO_{2}-Na_{2}WO_{4} (Mn-Ti-NW), the effect of loading of MnTiO_{3} or MnO_{x}-TiO_{2}, respectively, on two different supports (sol–gel SiO_{2} (SG) and commercial fumed SiO_{2} (CS)) was examined. The catalyst with the highest C_{2+} yield (21.6% with 60.8% C_{2}+ selectivity and 35.6% CH_{4} conversion) was 10 wt% MnTiO_{3}-NW/SG with an olefins/paraffin ratio of 2.2. The catalyst surfaces with low oxygen-binding energies were associated with high CH_{4} conversion. Stability tests conducted for over 24 h revealed that SG-supported catalysts were more durable than those on CS because the active phase (especially Na_{2}WO_{4}) was more stable in SG than in CS. With the use of SG, the activity of MnTiO_{3}-NW was not substantially different from that of Mn-Ti-NW, especially at high metal loading.},
keywords = {P08, TACO-associated},
pubstate = {published},
tppubtype = {article}
}

Shi, Junjie; Li, Hailian; Genest, Alexander; Zhao, Weixuan; Qi, Pengfei; Wang, Tao; Rupprechter, Günther
Journal ArticleOpen AccessIn: Applied Catalysis B: Environmental, vol. 301, pp. 120789, 2022.
Abstract | Links | BibTeX | Tags: P08
@article{Shi2022,
title = {High-performance water gas shift induced by asymmetric oxygen vacancies: Gold clusters supported by ceria-praseodymia mixed oxides},
author = {Junjie Shi and Hailian Li and Alexander Genest and Weixuan Zhao and Pengfei Qi and Tao Wang and Günther Rupprechter},
doi = {10.1016/j.apcatb.2021.120789},
year = {2022},
date = {2022-02-01},
urldate = {2022-02-01},
journal = {Applied Catalysis B: Environmental},
volume = {301},
pages = {120789},
publisher = {Elsevier BV},
abstract = {Modifying and controlling sites at the metal/oxide interface is an effective way of tuning catalytic activity, beneficial for bifunctional catalysis by reducible oxide supported \underline{metal nanoparticles}. We employed mixed ceria-praseodymia supported Au clusters for the \underline{water gas shift} reaction (WGSR). Varying the Ce:Pr ratio (4:1, 2:1, 1:4) not only allows to control the number of oxygen vacancies but, even more important, their local coordination, with asymmetrically coordinated O# being most active for water activation. These effects have been examined by X-ray absorption near edge structure (XANES), extended X-ray absorption fine structure (EXAFS), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, temperature programmed desorption/reduction (TPD/TPR), and density functional theory (DFT). Using the WGSR performance of Au/CeOx as reference, Au/Ce_{4}Pr_{1}O_{x} was identified to exhibit the highest activity, with a CO conversion of 75% at 300 °C, which is about 5-times that of Au/CeO_{x}. Au/Ce_{4}Pr_{1}O_{x} also showed excellent stability, with the conversion still being 70% after 50 h time-on-stream at 300 °C. Although a higher Pr content leads to more O vacancies, the catalytic activity showed a “volcano behavior”. Based on DFT, this was rationalized via the formation energy of oxygen vacancies, the binding energy of water, and the asymmetry of the O# site. The presented route of creating active vacancy sites should also be relevant for other heterogeneous catalytic systems.},
keywords = {P08},
pubstate = {published},
tppubtype = {article}
}

Kiatsaengthong, Danusorn; Jaroenpanon, Kanticha; Somchuea, Pooripong; Chukeaw, Thanaphat; Chareonpanich, Metta; Faungnawakij, Kajornsak; Sohn, Hiesang; Rupprechter, Günther; Seubsai, Anusorn
Journal ArticleOpen AccessIn: ACS Omega, vol. 7, no. 2, pp. 1785–1793, 2022.
Abstract | Links | BibTeX | Tags: P08
@article{Kiatsaengthong2022,
title = {Effects of Mg, Ca, Sr, and Ba Dopants on the Performance of La_{2}O_{3} Catalysts for the Oxidative Coupling of Methane},
author = {Danusorn Kiatsaengthong and Kanticha Jaroenpanon and Pooripong Somchuea and Thanaphat Chukeaw and Metta Chareonpanich and Kajornsak Faungnawakij and Hiesang Sohn and Günther Rupprechter and Anusorn Seubsai},
doi = {10.1021/acsomega.1c04738},
year = {2022},
date = {2022-01-04},
urldate = {2022-01-04},
journal = {ACS Omega},
volume = {7},
number = {2},
pages = {1785--1793},
publisher = {American Chemical Society (ACS)},
abstract = {Oxidative coupling of methane (OCM) is a reaction to directly convert methane into high value-added hydrocarbons (C_{2+}) such as ethylene and ethane using molecular oxygen and a catalyst. This work investigated lanthanum oxide catalysts for OCM, which were promoted with alkaline-earth metal oxides (Mg, Ca, Sr, and Ba) and prepared by the solution-mixing method. The synthesized catalysts were characterized using X-ray powder diffraction, CO_{2}-programmed desorption, and X-ray photoelectron spectroscopy. The comparative performance of each promoter showed that promising lanthanum-loaded alkaline-earth metal oxide catalysts were La-Sr and La-Ba. In contrast, the combination of La with Ca or Mg did not lead to a clear improvement of C_{2+} yield. The most promising LaSr50 catalyst exhibited the highest C_{2+} yield of 17.2%, with a 56.0% C_{2+} selectivity and a 30.9% CH_{4} conversion. Catalyst characterization indicated that their activity was strongly associated with moderate basic sites and surface-adsorbed oxygen species of O_{2}^{–}. Moreover, the catalyst was stable over 25 h at a reactor temperature of 700 °C.},
keywords = {P08},
pubstate = {published},
tppubtype = {article}
}
2021

Pramhaas, Verena; Rupprechter, Günther
Book ChapterIn: Ambient Pressure Spectroscopy in Complex Chemical Environments, vol. 1396, Chapter 6, pp. 119–145, American Chemical Society, 2021, ISBN: 9780841298125.
Abstract | Links | BibTeX | Tags: P08
@inbook{Pramhaas2021,
title = {Sum Frequency Generation in Ambient Environments: Vibrational Spectroscopy at Solid/Gas and Solid/Liquid Interfaces},
author = {Verena Pramhaas and Günther Rupprechter},
doi = {10.1021/bk-2021-1396.ch006},
isbn = {9780841298125},
year = {2021},
date = {2021-11-11},
booktitle = {Ambient Pressure Spectroscopy in Complex Chemical Environments},
journal = {ACS Symposium Series},
volume = {1396},
pages = {119--145},
publisher = {American Chemical Society},
chapter = {6},
abstract = {Molecules at solid/gas and solid/liquid interfaces are key players in many fields of technology, such as adsorption, corrosion, catalysis, electrochemistry and tribology. Their characterization is challenging, as they are “buried” under bulk phases. Due to its interface-specificity, nonlinear optical infrared-visible sum frequency generation (SFG) laser spectroscopy is an ideal method for their characterization, providing vibrational spectra of exclusively interfacial molecules. SFG also yields information on molecular structure, symmetry and orientation (tilt angle) and can be carried out with sub-picosecond time resolution. We introduce the SFG basics and instrumentation, and discuss exemplary studies chosen from recent research of gas adsorption on solid surfaces, as well as of increasingly complex molecules at solid/gas and solid/liquid interfaces.},
keywords = {P08},
pubstate = {published},
tppubtype = {inbook}
}

Haunold, Thomas; Rupprechter, Günther
LiOx-modification of Ni and Co3O4 surfaces: An XPS, LEIS and LEED study
Journal ArticleOpen AccessIn: Surface Science, vol. 713, pp. 121915, 2021.
Abstract | Links | BibTeX | Tags: P08
@article{Haunold2021,
title = {LiOx-modification of Ni and Co_{3}O_{4} surfaces: An XPS, LEIS and LEED study},
author = {Thomas Haunold and Günther Rupprechter},
doi = {10.1016/j.susc.2021.121915},
year = {2021},
date = {2021-11-01},
urldate = {2021-11-01},
journal = {Surface Science},
volume = {713},
pages = {121915},
publisher = {Elsevier BV},
abstract = {LiO_{x} was deposited at room temperature by physical vapor deposition (PVD) on polycrystalline Ni foil and Co_{3}O_{4}(111) thin film, creating uniform model systems well-suited for surface-sensitive characterization by X-ray photoelectron spectroscopy (XPS), low energy ion scattering (LEIS) or low energy electron diffraction (LEED). In the case of Ni, about 15 layers of LiO_{x} film were grown under the current conditions either stepwise or continuously, with XPS analysis indicating a deposition rate of 0.16 and 0.24 ML/min, respectively. Li 1s and O 1s spectra revealed that Li_{2}O and to a lesser extent LiOH were preferentially formed. The stability of the LiO_{x}films was examined in UHV, upon annealing at 573 K and upon hydrogen reduction at 723 K. On the more reactive Co_{3}O_{4}(111) film grown on Ir(100), the Li accommodation rate was about twice as high, at least within the first minutes of deposition. Post-deposition LEED showed an obscured cobalt oxide diffraction pattern, not unexpected in light of the LiO_{x} deposited. On both substrates, LEIS characterization of Li (≈ 103 eV) was prevented by the high background in this kinetic energy region, due to surface roughness and unspecific scattering. Still, LiO_{x} deposition was evident from the vanished LEIS signals of Ni or Co. The prepared LiO_{x}-modified surfaces may serve as starting point for the future growth of epitaxial Li_{x}CoO_{2} model systems.},
keywords = {P08},
pubstate = {published},
tppubtype = {article}
}

Zeininger, Johannes; Suchorski, Yuri; Raab, Maximilian; Buhr, Sebastian; Grönbeck, Henrik; Rupprechter, Günther
Single-Particle Catalysis: Revealing Intraparticle Pacemakers in Catalytic H2 Oxidation on Rh
Journal ArticleOpen AccessIn: ACS Catalysis, vol. 11, no. 15, pp. 10020–10027, 2021.
Abstract | Links | BibTeX | Tags: P08, TACO-associated
@article{Zeininger2021,
title = {Single-Particle Catalysis: Revealing Intraparticle Pacemakers in Catalytic H2 Oxidation on Rh},
author = {Johannes Zeininger and Yuri Suchorski and Maximilian Raab and Sebastian Buhr and Henrik Grönbeck and Günther Rupprechter},
doi = {10.1021/acscatal.1c02384},
year = {2021},
date = {2021-07-27},
urldate = {2021-07-27},
journal = {ACS Catalysis},
volume = {11},
number = {15},
pages = {10020--10027},
publisher = {American Chemical Society (ACS)},
abstract = {Self-sustained oscillations in H_{2} oxidation on a Rh nanotip mimicking a single catalytic nanoparticle were studied by in situ field emission microscopy (FEM). The observed spatio-temporal oscillations result from the coupling of subsurface oxide formation/depletion with reaction front propagation. An original sophisticated method for tracking kinetic transition points allowed the identification of local pacemakers, initiating kinetic transitions and the nucleation of reaction fronts, with much higher temporal resolution than conventional processing of FEM video files provides. The pacemakers turned out to be specific surface atomic configurations at the border between strongly corrugated Rh{973} regions and adjacent relatively flat terraces. These structural ensembles are crucial for reactivity: while the corrugated region allows sufficient oxygen incorporation under the Rh surface, the flat terrace provides sufficient hydrogen supply required for the kinetic transition, highlighting the importance of interfacet communication. The experimental observations are complemented by mean-field microkinetic modeling. The insights into the initiation and propagation of kinetic transitions on a single catalytic nanoparticle demonstrate how in situ monitoring of an ongoing reaction on individual nanofacets can single out active configurations, especially when combined with atomically resolving the nanoparticle surface by field ion microscopy (FIM).},
keywords = {P08, TACO-associated},
pubstate = {published},
tppubtype = {article}
}