Catalysis by ultrathin
LaBO3 (B=Co, Fe) perovskite films
Subproject P08
Perovskites are important catalysts, but detailed knowledge of their surface structure and chemistry is often lacking. The long-term objective of P08 is to elucidate structure-function correlations and visualize molecule-perovskite interaction in reactions involving O2, H2, CO, CO2, or H2O.
In the first project period, we will develop surface science-based model systems of LaCoO3 and LaFeO3 perovskites. Both epitaxial and polycrystalline thin films will be grown in UHV, guided by characterization via LEED, SXRD, SEM/EBSD, XPS/UPS/LEIS, IRAS, and TPD. Isotopically (18O or 13C) labeled adsorbates or films will reveal how oxygen and oxygen-containing molecules are activated. We will analyze the data in close collaboration with theoretical groups who simulate structure, stability, and infrared spectra (P03 Kresse).
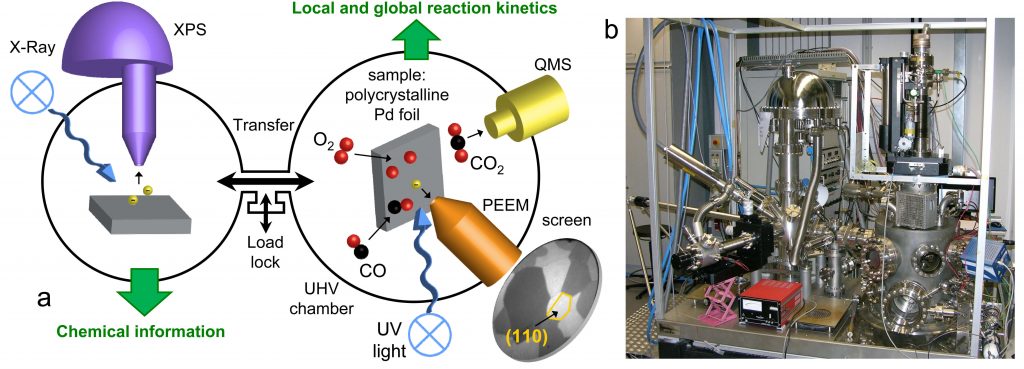
We will employ a unique combination of in situ surface spectroscopy (PM-IRAS, NAP-XPS, SXRD) and in situ surface microscopy (PEEM, SPEM), combined with MS gas phase analysis, to monitor ongoing reactions from HV to atmospheric pressure. This procedure should enable us to gain fundamental insights into the interplay of ternary oxide atomic and electronic structure, defects, composition, adsorption, as well as initiation and spatial progression of surface reactions on the mesoscale via reaction fronts (local kinetics by imaging). Project P08 will create the required bridge between single crystals (P02 Diebold, P04 Parkinson) and more application-relevant nanomaterials (P10 Föttinger).
Expertise
Our expertise is experimental surface science and its application to studies in heterogeneous catalysis. We operate a total of seven ultrahigh-vacuum (UHV) chambers, three of which are coupled to high-pressure cells. In situ and operando studies of surface reactions are carried out by area-averaging surface spectroscopy and real-time surface microscopy on the nano- and mesoscale. All chambers are equipped with facilities for sample preparation (sputtering, annealing, gas dosing), as well as various growth techniques (e-beam evaporators, Knudsen cells, sputter deposition). Analysis techniques used in our research include:
- Auger Electron Spectroscopy (AES)
- Field Emission Microscopy (FEM)
- Field Ion Microscopy (FIM)
- Gas Chromatography (GC)
- Low-Energy Electron Diffraction (LEED)
- Low-Energy Ion Scattering (LEIS)
- Mass Spectroscopy (MS)
- PhotoEmission Electron Microscopy (PEEM)
- Polarization Modulation Infrared Reflection-Absorption Spectroscopy (PM-IRAS)
- Sum Frequency Generation (SFG)
- Scanning PhotoElectron Microscopy (SPEM)
- Scanning Tunneling Microscopy (STM)
- Temperature-Programmed Desorption (TPD)
- Ultraviolet Photoelectron Spectroscopy (UPS)
- X-ray Absorption Spectroscopy (XAS)
- Surface X-Ray Diffraction (SXRD)
- X-ray Photoelectron Spectroscopy (XPS)
Collaboration Partners:
- Prof. Andreas Stierle, DESY Hamburg, Germany: SXRD
- Dr. Luca Gregoratti, ELETTRA Sincrotrone Trieste, Italy: SPEM
The combined application of photoemission electron microscopy (PEEM) and scanning photoelectron microscopy (SPEM) is particularly beneficial for TACO because these techniques allow visualizing ongoing reactions and local surface analysis on a µm-scale.
Team
Associates
Former Members
Publications
2016
Lukashuk, Liliana; Föttinger, Karin; Kolar, Elisabeth; Rameshan, Christoph; Teschner, Detre; Hävecker, Michael; Knop-Gericke, Axel; Yigit, Nevzat; Li, Hao; McDermott, Eamon; Stöger-Pollach, Michael; Rupprechter, Günther
Operando XAS and NAP-XPS studies of preferential CO oxidation on Co3O4 and CeO2-Co3O4 catalysts
Journal ArticleOpen AccessIn: Journal of Catalysis, vol. 344, pp. 1–15, 2016.
Abstract | Links | BibTeX | Tags: P08, P10, pre-TACO
@article{Lukashuk2016,
title = {Operando XAS and NAP-XPS studies of preferential CO oxidation on Co_{3}O_{4} and CeO_{2}-Co_{3}O_{4 }catalysts},
author = {Liliana Lukashuk and Karin Föttinger and Elisabeth Kolar and Christoph Rameshan and Detre Teschner and Michael Hävecker and Axel Knop-Gericke and Nevzat Yigit and Hao Li and Eamon McDermott and Michael Stöger-Pollach and Günther Rupprechter},
doi = {10.1016/j.jcat.2016.09.002},
year = {2016},
date = {2016-12-01},
urldate = {2016-12-01},
journal = {Journal of Catalysis},
volume = {344},
pages = {1--15},
publisher = {Elsevier BV},
abstract = {Co_{3}O_{4} is a promising catalyst for removing CO from H_{2} streams via the preferential CO oxidation (PROX). A Mars-van-Krevelen redox mechanism is often suggested but a detailed knowledge especially of the oxidation state of the catalytically active surface under reaction conditions is typically missing. We have thus utilized operando X-ray absorption spectroscopy to examine structure and oxidation state during PROX, and near atmospheric pressure-XPS at low photoelectron kinetic energies and thus high surface sensitivity to monitor surface composition changes. The rather easy surface reduction in pure CO (starting already at ∼100 °C) and the easy reoxidation by O_{2} suggest that molecularly adsorbed CO reacts with lattice oxygen, which is replenished by gas phase O_{2}. Nevertheless, the steady state concentration of oxygen vacancies under reaction conditions is too low even for XPS detection so that both the bulk and surface of Co_{3}O_{4} appear fully oxidized during PROX. Furthermore, the effect of adding CeO_{2} (a less active material) to Co_{3}O_{4} was studied. Promotion of Co_{3}O_{4} with 10 wt% CeO_{2} increases the reduction temperatures in CO and H_{2} and enhances the PROX activity. Since CeO_{2} is a less active material, this can only be explained by a higher activity of the Co-O-Ce interface.},
keywords = {P08, P10, pre-TACO},
pubstate = {published},
tppubtype = {article}
}

Wolfbeisser, Astrid; Sophiphun, Onsulang; Bernardi, Johannes; Wittayakun, Jatuporn; Föttinger, Karin; Rupprechter, Günther
Methane dry reforming over ceria-zirconia supported Ni catalysts
Journal ArticleOpen AccessIn: Catalysis Today, vol. 277, pp. 234–245, 2016.
Abstract | Links | BibTeX | Tags: P08, P10, pre-TACO
@article{Wolfbeisser2016,
title = {Methane dry reforming over ceria-zirconia supported Ni catalysts},
author = {Astrid Wolfbeisser and Onsulang Sophiphun and Johannes Bernardi and Jatuporn Wittayakun and Karin Föttinger and Günther Rupprechter},
doi = {10.1016/j.cattod.2016.04.025},
year = {2016},
date = {2016-11-15},
urldate = {2016-11-15},
journal = {Catalysis Today},
volume = {277},
pages = {234--245},
publisher = {Elsevier BV},
abstract = {Nickel nanoparticles supported on Ce_{1-x}Zr_{x}O_{2} mixed oxides prepared by different synthesis methods, as well as Ni-ZrO_{2} and Ni-CeO_{2}, were evaluated for their catalytic performance in methane dry reforming (MDR). MDR is an interesting model reaction to evaluate the reactivity and surface chemistry of mixed oxides. Textural and structural properties were studied by N_{2} adsorption and XRD. Mixed oxide preparation by co-precipitation resulted in catalysts with higher surface area than that of pure ZrO_{2} or CeO_{2}. XRD analysis showed the formation of different Ce_{1-x}Zr_{x}O_{2} solid solutions depending on using a surfactant or not. The catalyst prepared by surfactant assisted co-precipitation was not active for methane dry reforming most likely because of the encapsulation of Ni particles by ceria-zirconia particles, as revealed by TEM and H_{2} chemisorption. The catalytic activity of the catalyst prepared by co-precipitation without surfactant was comparable to Ni-ZrO_{2}. Clearly, catalyst activity strongly depends on preparation and on the resulting phase composition rather than on nominal composition. Compared to Ni-ZrO_{2} the ceria-zirconia supported Ni catalyst did not achieve higher activity or stability for methane dry reforming but, nevertheless, the formation of filamentous carbon was strongly reduced (100 times less carbonaceous species). Consequently, using ceria-zirconia as a support material decreases the risk of reactor tube blocking.},
keywords = {P08, P10, pre-TACO},
pubstate = {published},
tppubtype = {article}
}
2014

Föttinger, Karin; Rupprechter, Günther
Journal ArticleIn: Accounts of Chemical Research, vol. 47, no. 10, pp. 3071–3079, 2014.
Abstract | Links | BibTeX | Tags: P08, P10, pre-TACO
@article{Foettinger2014,
title = {In Situ Spectroscopy of Complex Surface Reactions on Supported Pd–Zn, Pd–Ga, and Pd(Pt)–Cu Nanoparticles},
author = {Karin Föttinger and Günther Rupprechter},
doi = {10.1021/ar500220v},
year = {2014},
date = {2014-09-23},
journal = {Accounts of Chemical Research},
volume = {47},
number = {10},
pages = {3071--3079},
publisher = {American Chemical Society (ACS)},
abstract = {It is well accepted that catalytically active surfaces frequently adapt to the reaction environment (gas composition, temperature) and that relevant “active phases” may only be created and observed during the ongoing reaction. Clearly, this requires the application of in situ spectroscopy to monitor catalysts at work. While changes in structure and composition may already occur for monometallic single crystal surfaces, such changes are typically more severe for oxide supported nanoparticles, in particular when they are composed of two metals. The metals may form ordered intermetallic compounds (e.g. PdZn on ZnO, Pd_{2}Ga on Ga_{2}O_{3}) or disordered substitutional alloys (e.g. PdCu, PtCu on hydrotalcite). We discuss the formation and stability of bimetallic nanoparticles, focusing on the effect of atomic and electronic structure on catalytic selectivity for methanol steam reforming (MSR) and hydrodechlorination of trichloroethylene. Emphasis is placed on the in situ characterization of functioning catalysts, mainly by (polarization modulated) infrared spectroscopy, ambient pressure X-ray photoelectron spectroscopy, X-ray absorption near edge structure, and X-ray diffraction. In the present contribution, we pursue a two-fold, fundamental and applied, approach investigating technologically applied catalysts as well as model catalysts, which provides comprehensive and complementary information of the relevant surface processes at the atomic or molecular level. Comparison to results of theoretical simulations yields further insight.
Several key aspects were identified that control the nanoparticle functionality: (i) alloying (IMC formation) leads to site isolation of specific (e.g. Pd) atoms but also yields very specific electronic structure due to the (e.g. Zn or Ga or Cu) neighboring atoms; (i) for intermetallic PdZn, the thickness of the surface alloy, and its resulting valence band structure and corrugation, turned out to be critical for MSR selectivity; (ii) the limited stability of phases, such as Pd_{2}Ga under MSR conditions, also limits selectivity; (iii) favorably bimetallic catalysts act bifunctional, such as activating methanol AND water or decomposing trichlorothylene AND activating hydrogen; (iv) bifunctionality is achieved either by the two metals or by one metal and the metal–oxide interface; (v) intimate contact between the two interacting sites is required (that cannot be realized by two monometallic nanoparticles being just located close by).
The current studies illustrate how rather simple bimetallic nanoparticles may exhibit intriguing diversity and flexibility, exceeding by far the properties of the individual metals. It is also demonstrated how complex reactions can be elucidated with the help of in situ spectroscopy, in particular when complementary methods with varying surface sensitivity are applied.},
keywords = {P08, P10, pre-TACO},
pubstate = {published},
tppubtype = {article}
}
Several key aspects were identified that control the nanoparticle functionality: (i) alloying (IMC formation) leads to site isolation of specific (e.g. Pd) atoms but also yields very specific electronic structure due to the (e.g. Zn or Ga or Cu) neighboring atoms; (i) for intermetallic PdZn, the thickness of the surface alloy, and its resulting valence band structure and corrugation, turned out to be critical for MSR selectivity; (ii) the limited stability of phases, such as Pd2Ga under MSR conditions, also limits selectivity; (iii) favorably bimetallic catalysts act bifunctional, such as activating methanol AND water or decomposing trichlorothylene AND activating hydrogen; (iv) bifunctionality is achieved either by the two metals or by one metal and the metal–oxide interface; (v) intimate contact between the two interacting sites is required (that cannot be realized by two monometallic nanoparticles being just located close by).
The current studies illustrate how rather simple bimetallic nanoparticles may exhibit intriguing diversity and flexibility, exceeding by far the properties of the individual metals. It is also demonstrated how complex reactions can be elucidated with the help of in situ spectroscopy, in particular when complementary methods with varying surface sensitivity are applied.